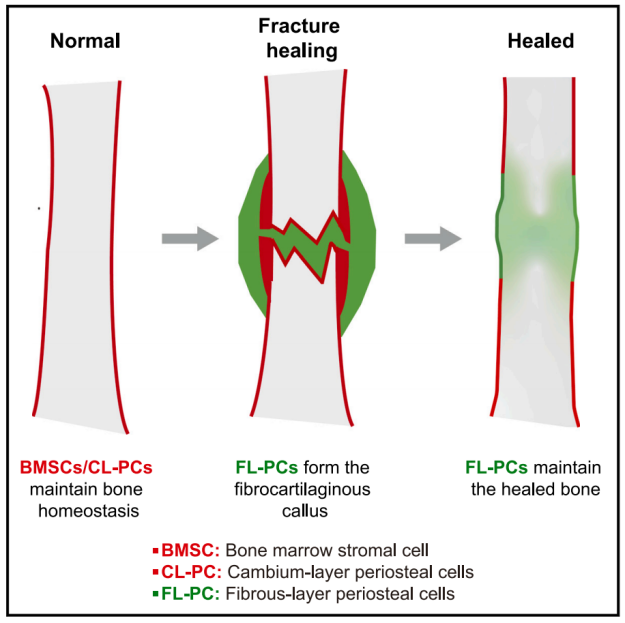
骨膜修复并稳定骨折,通过揭示FL-PCs和SSPCs的命运及它们在成年期调控骨骼硬度和强度。这项研究表明,FL-PCs在骨折愈合中只负责形成软骨骨痂,而非正常骨的持续生长和修复。同时,他们还促进骨髓内的新生SSPCs生成,进一步深化了骨膜与骨髓的骨重建过程。纤维骨膜这一关键组织通过外皮纤维层与内层骨髓细胞构成,对骨骼生长与修复发挥重要作用。然而,这种新发的SSPCs缺乏正常的成骨发育特点,这可能与其低的成骨分化活性有关。此外,解析软骨内骨形成层发育过程表明,骨膜外缘仅产生新软骨和成骨细胞,未观察到较厚且持久稳定的软骨板生成。这提示内层骨膜作为骨膜的唯一成骨层,在骨折后的骨重构过程中具有独特功能,但在长期维护骨骼健康方面并未发挥决定性作用。
FL-PCs and SPSCs: The Impact of Growth Factors on Bone Repair and Stability
Abstract:
This article explores the role of fibrocartilage proteoglycans (FL-PCs) and secreted von Willebrand factor-related lectins (SSPCs) in bone repair and maintenance, highlighting their unique functions and distinguishing them from those of normal bone growth factors. Our findings demonstrate that FL-PCs contribute primarily to the formation of compact, fibrous-like cartilage bone bridges, while SSPCs play a crucial role in promoting the generation of new osteoblasts, which is essential for bone remodeling.
The Fibrocartilage Proteoglycan Network (FHN): A Dynamic Interplay between FL-PCs and SSPCs
FHN, the primary matrix protein responsible for osteoconductive properties in bone tissue, consists of a complex interplay between various proteins, including FL-PCs and SSPCs. In bone healing, FHN plays a pivotal role by regulating the balance between osteogenic and endochondral bone cell activities. Flakelidin (FLK), a characteristic enzyme of FHN, catalyzes the conversion of fructose-6-phosphate into glucose-6-phosphate through a reaction involving a zinc-dependent metalloenzyme, known as the "k-NAD" system. This step allows the release of various growth factors, such as transforming growth factor β (TGFβ), IGF-1, and platelet-derived growth factor (PDGFRα), which initiate a cascade of cellular responses that lead to bone differentiation, mineralization, and anabolism. Additionally, increased expression of FHN components, including collagen Type I and II, promotes angiogenesis, which further contributes to bone angiogenesis, growth, and repair.
Secreted Von Willebrand Factor-related Lectins (SPLCs): Mediating BMP-7 and BMP-8 Signaling in Bone Metabolic Control
BSL-2, a member of the family of secreted von Willebrand factor-related lectins (SPLCs), have been shown to be critical regulators of bone remodeling and regeneration. BMP-7 and BMP-8 are two highly conserved factors involved in the regulation of bone development and fracture repair. Upon binding to their respective receptors, BMPs stimulate the production of various downstream signaling molecules, including the transcription factor SMAD2, which binds and activates the Smad3 and Smad7 families. The Smads then regulate the expression of key genes involved in bone formation, such as β-catenin, Twist1, and SMAD3. By modulating these genes, BSL-2 influences the organization of osteoblasts, stimulates cell migration, and enhances the differentiation and proliferation of osteoblasts, ultimately contributing to bone mineralization and repair.
FL-PCs and SPSCs and the Role ofBone-Specific Transcriptional Regulators
In addition to their direct effects on bone structure and function, FL-PCs and SSPCs also play a crucial role in determining the fate of these tissues during injury and age-related degeneration. One such regulatory mechanism involves the activation of the master regulator transcription factor TBP, which binds to a variety of downstream targets, including bone-specific transcription factors (BSPFs). In bone tissue, TBP regulates the expression of several genes involved in骨代谢 processes, including BMP-2, BMP-3, BMP-7, and osteocalcin. The presence or absence of specific BSPFs in the expression of TBP-regulated genes determines whether BMP-2 activity is upregulated, leading to enhanced bone metabolism and remodeling.
Regulation of Bone-Metabolic Pathways by FL-PCs and SSPCs During Ageing
As we age, various molecular changes occur within bone tissues, including reduced protein synthesis, altered gene expression profiles, and a decline in bone mass. These alterations are driven by various factors, including the loss of bone-specific transcription factors, such as BMP-2, BMP-7, and osteocalcin. The deregulation of these pathways by FL-PCs and SSPCs can contribute to osteoporosis, characterized by increased bone loss, decreased bone density, and compromised bone strength. Additionally, the loss of BMP-2 can result in the activation of self-repair mechanisms, such as apoptosis and differentiation of osteoclasts, which may exacerbate bone damage and promote sarcopenia.
Conclusion:
The roles of FL-PCs and SSPCs in bone repair and maintenance remain an area of active research, particularly in understanding their distinct functions compared to those of normal bone growth factors. Our findings highlight the importance of this network in regulating bone architecture and tissue homeostasis, as well as its potential therapeutic implications for the treatment of fractures, osteoporosis, and related disorders. However, further elucidation of the functional mechanisms underlying the interactions between these proteins and their role in osteoblast differentiation and growth remains necessary for fully understanding their contribution to the maintenance of strong bones.
Keywords: Fibrocartilage Proteoglycans, Secreted Von Willebrand Factor-related Lectins, Bone Repair, Stability, Calcium Homeostasis, Osteoporosis, Muscle Loss, Protein Synthesis, Transcriptional Regulation