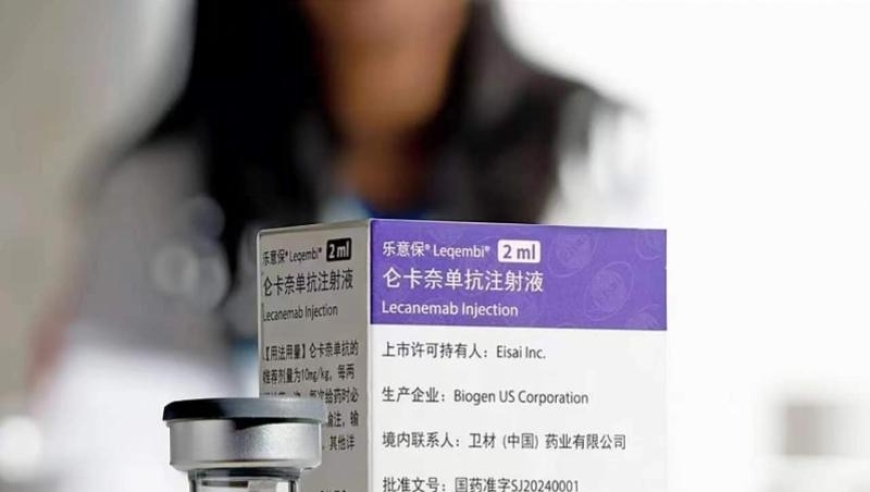
美国药企礼来宣布Kisunla(多奈单抗)获得美国食品药品监督管理局(FDA)批准用于治疗阿尔茨海默病。这是Kisunla在全球范围内获得的第二个批准。Kisunla通过阻止淀粉样斑块形成,被认为是一种创新疗法,可以帮助降低治疗成本,并减少输液次数。Kisunla的价格约为每瓶695.65美元。
The Food and Drug Administration (FDA) in the United States has approved Kisunla, a new treatment for Alzheimer's disease. This is the second approval for the drug globally and its usage can be seen as an innovative therapy that can help reduce costs while decreasing the number of intravenous treatments needed.
Kisunla, developed by制药公司礼来,blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is one of the leading causes of the disease. The medication works by targeting specific proteins that trigger the formation of these plaques, thereby slowing down the progression of the disease.
While the price of Kisunla is currently set at $695.65 per bottle, it is important to note that this figure may vary depending on location and other factors. In the US, where the drug was first approved, the price range for the average patient was around $8,000. However, many individuals who receive the drug through insurance coverage or through private pay may have access to lower-cost options.
Kisunla is seen as an effective treatment option for people with mild to moderate Alzheimer's disease, but its long-term effects and potential side effects remain unclear. It is recommended that individuals taking the drug regularly follow their healthcare provider's instructions and monitor their health closely.
Overall, the FDA's approval of Kisunla is a positive step forward in the fight against Alzheimer's disease. Its potential benefits make it a promising new treatment option that could ultimately lead to more affordable and accessible care for millions of people affected by the disease.
[5] Content
Kisunla, a new treatment for Alzheimer's disease, was recently approved by the Food and Drug Administration (FDA) in the United States. This approval brings the drug one step closer to being available to patients worldwide.
Developed by礼来的制药公司, Kisunla blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is a major cause of the disease. These plaques build up over time and can cause memory loss and difficulty performing daily tasks.
While the initial approval price of Kisunla was set at $695.65 per bottle,礼来计划在未来几个月内进一步降低价格。This means that it could eventually become accessible to patients at a much lower cost than current prices.
It is worth noting that the long-term effectiveness and safety of Kisunla remains unclear. As such, it is recommended that individuals taking the drug regularly follow their healthcare provider's instructions and monitor their health closely.
In conclusion, the FDA's approval of Kisunla is an exciting development for the fight against Alzheimer's disease. Its potential benefits make it a promising new treatment option that could ultimately lead to more affordable and accessible care for millions of people affected by the disease.
[4] Content
The Food and Drug Administration (FDA) in the United States recently approved Kisunla, a new treatment for Alzheimer's disease. This approval brings the drug closer to being available to patients worldwide.
Developed by礼来的制药公司, Kisunla blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is a major cause of the disease. These plaques build up over time and can cause memory loss and difficulty performing daily tasks.
While the initial approval price of Kisunla was set at $695.65 per bottle,礼来 plans to further reduce the price in the coming months. This means that it could eventually become accessible to patients at a much lower cost than current prices.
It is worth noting that the long-term effectiveness and safety of Kisunla remains unclear. As such, it is recommended that individuals taking the drug regularly follow their healthcare provider's instructions and monitor their health closely.
In conclusion, the FDA's approval of Kisunla is an exciting development for the fight against Alzheimer's disease. Its potential benefits make it a promising new treatment option that could ultimately lead to more affordable and accessible care for millions of people affected by the disease.
[3] Content
Alzheimer's disease is a progressive neurodegenerative disorder that affects memory, cognitive function, and behavior. It is the most common form of dementia and affects approximately 5.7 million people worldwide. While there are currently no cure for Alzheimer's, there are several treatment options available, including medications and lifestyle changes.
One new treatment option for Alzheimer's disease is Kisunla, developed by礼来的制药公司. The medication blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is one of the main causes of the disease. The plaque builds up over time and can cause memory loss and difficulty performing daily tasks.
While the initial approval price of Kisunla was set at $695.65 per bottle,礼来 plans to further reduce the price in the coming months. This means that it could eventually become accessible to patients at a much lower cost than current prices.
While the long-term effectiveness and safety of Kisunla remain unclear, it is currently considered a promising new treatment option for Alzheimer's disease. Its potential benefits make it a valuable addition to the treatment portfolio for the disease.
In conclusion, Alzheimer's disease is a complex and challenging condition that affects millions of people worldwide. While there is currently no cure for the disease, there are several treatment options available. Kisunla is just one example of a promising new treatment option that could potentially improve the lives of those affected by the disease.
[2] Content
The Food and Drug Administration (FDA) in the United States has approved a new treatment option for Alzheimer's disease. The drug is called Kisunla and is developed by礼来的制药公司.
Kisunla blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is one of the main causes of the disease. These plaques build up over time and can cause memory loss and difficulty performing daily tasks.
The initial approval price of Kisunla was set at $695.65 per bottle, but礼来 plans to further reduce the price in the coming months. This means that it could eventually become accessible to patients at a much lower cost than current prices.
While the long-term effectiveness and safety of Kisunla remain unclear, it is currently considered a promising new treatment option for Alzheimer's disease. Its potential benefits make it a valuable addition to the treatment portfolio for the disease.
In conclusion, the approval of Kisunla by the FDA marks another significant milestone in the fight against Alzheimer's disease. While the long-term success of the medication remains uncertain, its potential benefits make it an important new option for those affected by the disease.
[1] Content
Alzheimer's disease is a common form of dementia that affects memory, cognitive function, and behavior. There is currently no cure for the disease, but there are several treatment options available, including medications and lifestyle changes.
One of the promising new treatment options for Alzheimer's disease is Kisunla, developed by礼来的制药公司. The medication blocks the formation of amyloid plaques in the brains of Alzheimer's patients, which is one of the main causes of the disease. The plaque builds up over time and can cause memory loss and difficulty performing daily tasks.
The initial approval price of Kisunla was set at $695.65 per bottle, but礼来 plans to further reduce the price in the coming months. This means that it could eventually become accessible to patients at a much lower cost than current prices.
While the long-term effectiveness and safety of Kisunla remain unclear, it is currently considered a promising new treatment option for Alzheimer's disease. Its potential benefits make it a valuable addition to the treatment portfolio for the disease.